What is GxP assessment?

GxP Assessment determines whether a system requires a validation to identify whether the system has a GxP impact. Is the system is used to monitor, control or supervise a GxP manufacturing or packaging process and have the potential to affect Product Quality, Safety, Identity or Efficacy?
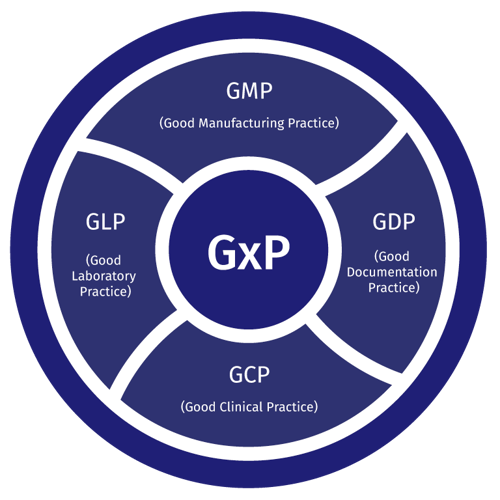
Types of GxP
The ‘x’ represents a field of focus for Good Practice compliance standards. Good Manufacturing Practices (GMP) ensure packages and products are labeled correctly, are uncontaminated and meet intended claims. Good Documentation Practices (GDP) maintain product quality throughout all stages of the supply chain. Good Laboratory Practices (GLP) promote the development of quality and reliable test data. Good Clinical Practices (GCP) ensure subject rights are protected and that data is credible in clinical trials.
The result of the GxP Assessment and system Category checklist decided whether Validation required for computerized system.
Risk Assessment
During software testing, functional software system processes that are governed by GxP regulations must be identified. An assessment should be performed to address the probability of risk (probability of occurrence and/or detection) for each GxP functional process (or group of processes) to determine a proper risk priority, which in turn, will define the testing strategy. Arbour Group can assist not only in conducting a Risk Assessment to identify key risk consequences, but also help in mitigating the GxP regulations that affect testing. Determine the prioritization of fixes to a system and when it is acceptable to release a system with support from Arbour Group.
If Outcome is “GxP Applicable” then follow Computer system validation procedure and other risk assessment for validating the system.
GxP compliance checklist
Use this GxP audit checklist below to gauge your business’s ability to meet the core and generic requirements of GxP. If you can answer ‘yes’ to each question, your business has a firm GxP base with which you can tackle your industry-specific ‘x’ requirements.
All systems which handle data or processes which fall under the impact of GxP or any other regulatory requirements must be validated
Determine whether the application has been validated elsewhere you in organization. The amount of work required may be significantly reduced by referring some of the deliverables in as in state or with slight changes. The details must be addressed in the change control.