Food and Drug Administration (FDA) of the United States- Import Requirements

Before entering the United States, all FDA-regulated products are electronically checked. The FDA may deny entry if it appears that the product doesn’t satisfy the law of the United States. PREDICT, an FDA screening program, and the U.S. ACE programme at Custom demands more meticulousness. Incorrect entered data might result in fines and stricter data verification requirements. Getting a problem fixed after it occurred can be frustrating. U.S. and FDA legal authorities To determine the product’s entry, Customs and Border Patrol (CBP) rely on each other’s import regulations. Importers can prevent expensive mistakes and delays by understanding the rules.
Entry Submission for FDA-Regulated Products
The import broker must notify CBP of the entry as the initial step in any importation process. Tariffs, taxes, and other charges are gathered by CBP for the goods.
- Open an account with CBP
- Collaborate with an import broker who is knowledgeable about FDA imports and ideally, your particular product.
- Provide accurate documentation for each shipment.
- Use a commercial invoice from CBP.
- Maintain thorough and accurate records
- Create efficient import processes to handle the regulatory issues
Complying with FDA Regulations
The FDA import programme is governed by the Federal Food, Drug, and Cosmetic Act (FDCA), as modified (21 U.S.C. Part 381). Several other laws that the FDA is related to increase its legal authority in the following areas:
Examples comprise:
- Fair Packaging and Labelling Act
- Radiological Protection and Health Act
- Act on Public Health Services
- Act on Comprehensive Tobacco Health and Education
- Food Safety Act of FDA
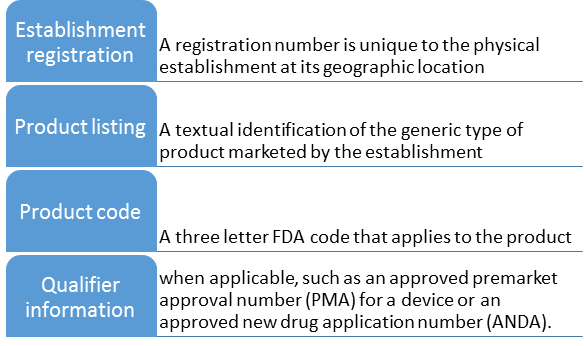
The original importer establishment must be registered with FDA in order to operate. Initiating contact with the legal authorities is the responsibility of the initial importer and the U.S. import broker.
When a product is contaminated or misbranded in some way, it is judged to be in violation:
The FDA notifies the initial importer or the consignee of the precise reason or misbranding allegation if the goods looks to be in violation of FDA law.
A claim of adulteration will reveal a defect in the product itself.
Examples comprise:
- There are bacteria on the product. i.e. contamination
- Consists entirely or partially of any unclean, rotten, or decayed material that was prepared or stored in contaminated conditions
- The manufacturing processes and quality standards fall short of the strength, quality, or purity it promises to have
Misbranding charges examples include:
- false or deceptive labelling in any situation
- deceptive or false advertisement
- Information on the label is missing
- inadequate usage instructions
- Unregistered business
The FDA’s import requirements are:
FDA Import Procedure at First
The establishment registration cost must be paid before the FDA import procedure may be started. The FDA will send an email confirming that the registration is finished if all the requirements are met.
The FDA’s import computer programme will immediately hold the cargo if the information is incorrect or insufficient. The requirements for each piece of information should be consistent with the rest of the requirements. The importer and broker should speak with the District’s import officer, who is in charge of the port of entry, immediately if the merchandise is detained. Recurrent detentions can be expensive. The importer, his broker, CBP, and FDA officials may find it frustrating. In addition, there are long-term effects.
Documents Needed by the FDA
- the Bill of Lading (BOL)
- Airway Bill (AWB), a statement
- order for purchase
Where appropriate, the importer should also provide the FDA with the commodity-specific certifications (USDA permits, Impact Resistance test results, etc.), packing list/growers list, copies of labelling, documentation identifying the real manufacturer, documentation describing the reasons why goods labelled as “U.S. Goods Returned” are being sent back, certificate of analysis, intended use statement or End-Use Statement, and some other pertinent documentation requested. The FDA should additionally receive CBP forms 3461 and/or 7501 for manual (non-ABI) entries.